No products in the cart.
Medium and High Binding Well Plates
Applications & TechniquesBiomat polystyrene Medium- and High-Binding well plates are ideal to coat biomolecules and set up ELISA tests to be used for diagnostics and in the pharmaceutical field.
The enzyme-linked immunosorbent assay (ELISA) is one of the most sensitive and reproducible technologies available. The assay you can set up is rapid, simple to perform, and easily automated.
Polystyrene is composed of an aliphatic carbon chain with pendant intermittent benzene rings. This provides a very hydrophobic surface, and plates of this type are typically referred to as “medium binding”. Thanks to a surface treatment such as irradiation, it is possible to enhance the polystyrene plates surface’s binding capacity. This kind of surface treatment breaks a certain number of benzene rings, yielding carboxyl (COOH) and hydroxyl (OH) groups. The presence of these molecular groups allows for hydrophilic interactions. Well plates with such characteristics are referred to as “high binding”. The resulting surface is primarily hydrophobic, with intermittent carboxylic and hydroxylic groups capable of ionic interactions with positively charged groups on biomolecules.
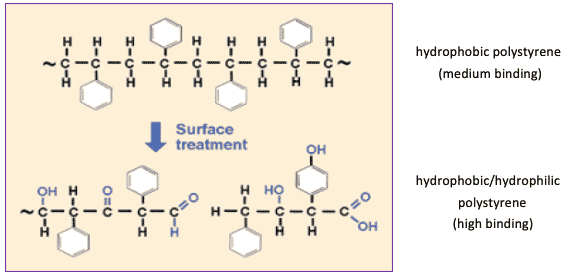
The forces that passively adsorb biomolecules onto the surface of Medium-Binding and High-Binding plates are typically:
- hydrophobic interactions
- van der Waals forces
- hydrogen bonding
- ionic interactions
The following picture shows the four physical adsorptions that occur when a biomolecule comes into contact with the bottom of a polystyrene plate.

Selecting the best type of well plates (high-binding or medium-binding) to adsorb the biomolecule requires knowing the molecule’s chemical structure in order to exploit its interaction with the well surface.
The 20 amino acids that make up the primary structure of a protein can be divided into two categories:
- Hydrophobic amino acids, such as glycine, alanine, valine, leucine, isoleucine, proline, phenylalanine, methionine, showed a better binding capacity with Medium-Binding 96 Well Plates.
- Polar/charged amino acids, such as tyrosine, tryptophan, serine, threonine, arginine, histidine, lysine, aspartic acid, glutamic acid, showed a better binding capacity with High-Binding 96 Well Plates.
Medium and high binding well plates tests
Below are some examples of different molecules coatings on Biomat Medium- and High-Binding well plates, especially using proteins with a known amino acid composition.
Adsorption of Bovine Serum Albumin
Biotinylated Bovine Serum Albumin at 5 µg/ml was coated on Biomat Medium- and High-Binding 96-well plates.
After a post coating step, plates were evaluated for their biotin activity level. At the same time, microplates treated only with post coating tested blank.
BSA-biotin Medium- and High-Binding coated and uncoated wells were incubated with 100 µl of streptavidin-peroxidase 1 mg/ml diluted at a 1:70,000 ratio for 30 minutes at room temperature.
After a washing step, the wells were incubated with TMB for 10 minutes at room temperature and blocked with sulfuric acid 1N.
mO.D. values were read at 450nm and are shown in the below diagram.
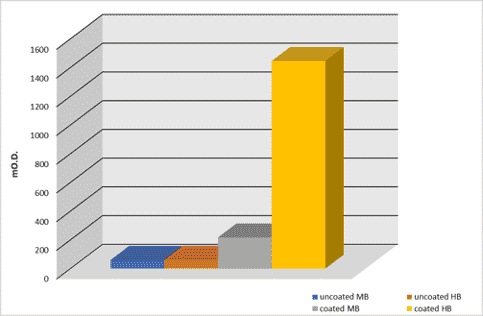
Results
Results show that biotinylated albumin is more clearly adsorbed onto a high binding surface than onto a medium binding surface. Medium-binding surfaces absorb about 8less biotinylated albumin compared to high-binding surfaces.
In fact, if we analyze the amino acid composition of the of Bovine Serum Albumin we find that 65% is composed of polar/charged (hydrophilic) amino acids and 35% of hydrophobic amino acids.
The prevalence of hydrophilic amino acids allows to achieve a more marked bond on the high-binding surface exploiting ionic interactions and van der Waals forces.
From the analysis of protein structures, we also know that hydrophobic residues such as Val, Leu, Ile, Phe, and Met generally tend to be buried inside, and polar side chains exposed to solvent.
Adsorption of human IgG
Human IgG at 2.5 µg/ml were coated on Biomat Medium- and High-Binding 96-well plates.
After a post-coating step, microplates were evaluated for their IgG binding activity. At the same time, plates treated only with post coating tested blank.
Human IgG Medium- and High-Binding coated and uncoated wells were incubated with 50 µl of αHuman IgG-peroxidase 1 mg/ml diluted at a 1:400,000 ratio for 1 hour at room temperature.
After a washing step, the wells were incubated with TMB for 10 minutes at room temperature and blocked with sulfuric acid 1N.
mO.D. values were read at 450nm and are shown in the below diagram.
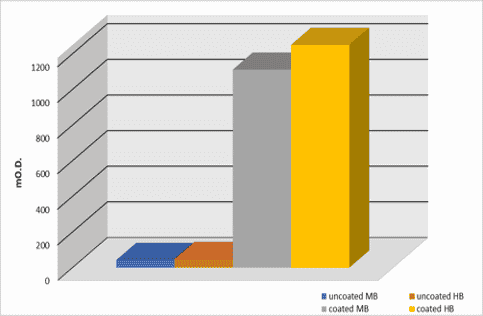
Results
Results show that human IgGs are almost equally adsorbed on a medium-binding and a high-binding surface. This occurs because the amino acid composition of the IgG’s primary structure is approximately 50% hydrophobic amino acids and 50% polar/charged (hydrophilic) amino acids. Moreover, in the IgG’s primary structure there are large thickenings of hydrophobic amino acids, which can justify the bonds to the medium-binding surface.
Our test showed that both Medium- and High-Binding well plates surfaces adsorb an equivalent amount of antibody.
Another factor influencing the test sensitivity is the IgG’s steric position after the coating step, which requires further analysis. The IgG ideal position requires having the Fc part facing the well surface and the F(ab)2 part free to bind to the antigen. To choose the most suitable surface for your test you must patiently test both.
Adsorption of Poly-DL-alanine (M.W. 1,000-5,000)
Biotinylated Poly-DL-alanine, at 20 µg/ml, was coated on Biomat Medium- and High-Binding 96-well plates. After a post coating step, plates were evaluated for their biotin activity. At the same time, plates treated only with post-coating tested blank.
Poly-DL-alanine – biotin medium and high-binding coated and uncoated wells were incubated with 100 µl of Streptavidin-Peroxidase 1 mg/ml diluted at a 1:60,000 ratio for 30 minutes at room temperature (RT).
After a washing step, the wells were incubated with TMB for 15 minutes at RT and blocked with sulfuric acid 1N.
mO.D. values were read at 450nm and are shown in the below diagram.

Results
Results show that biotinylated Poly-DL-alanine is more clearly adsorbed onto a medium-binding surface than onto a high-binding surface. High-binding surfaces absorb about biotinylated Poly-DL-alanine compared to medium-binding surfaces.
In fact, since proline is a non-polar amino acid, it is better absorbed by a completely hydrophobic surface such as a medium-binding one.
The presence of completely hydrophobic amino acids allows for a better bonding to medium-binding surfaces, exploiting hydrophobic interactions.
In our test, the proline polymer was our example of a hydrophobic molecule.